HepatiCan
Making a transformative and disruptive change to the care of patients with acute/severe liver failure.

Overview
1 million people die each year from liver disease (Acute and Chronic Liver Failure) Worldwide and only 1 in 10 people who need a liver transplant will get one.
Hepatican Limited has developed a novel ATMP and device – BioArtificial Liver - to rescue patients with Acute Liver Failure (‘ALF’). ALF is an unmet medical need with an addressable market in Europe of £5.7 – 7 billion.
The HepatiCan system employs a cryo-preservable, single use cartridge, which means treatment can be initiated in 24h unlike other cell therapy solutions. The HepatiCan system’s preclinical data has delivered proof of concept with the product at clinical scale and has received Orphan Drug Designation (ODD) in EMA and FDA jurisdictions.
Landscape
Hepatican Limited is a UK SME recently spun out of University College London.
After more than 20 years or research and development we have a Bio-artificial liver cryopreserved in a canister (HepatiCan) almost ready for first-in-man trials. Concept has been proven in pig model.
HepatiCan delivers liver functions on demand from a cryopreserved canister containing approximately 70 billion human liver cells. Two canisters are expected to be sufficient to buy enough time for the patient’s own liver to recover or to be transplanted.
HapatiCan is classified by MHRA as a combined ATMP and Medical Device.

Indications
Initial trial will be in Acute Liver Failure (ALF) patients.
ALF is a growing unmet medical need.
Currently the only life saving therapy is a liver transplant. There are only enough livers available to transplant 1 in 10 patients with liver failure.
1 in 3 patients on the UK liver transplant waiting list die whilst waiting.
Further indications for HepatiCan are:
As a bridge to transplant allowing patient to survive until suitable match.
To provide liver function if transplanted liver fails to function and needs kickstarting or retransplant.
For Acute on Chronic Liver Failure
To enable a heavily resected liver to recover after tumour excision.
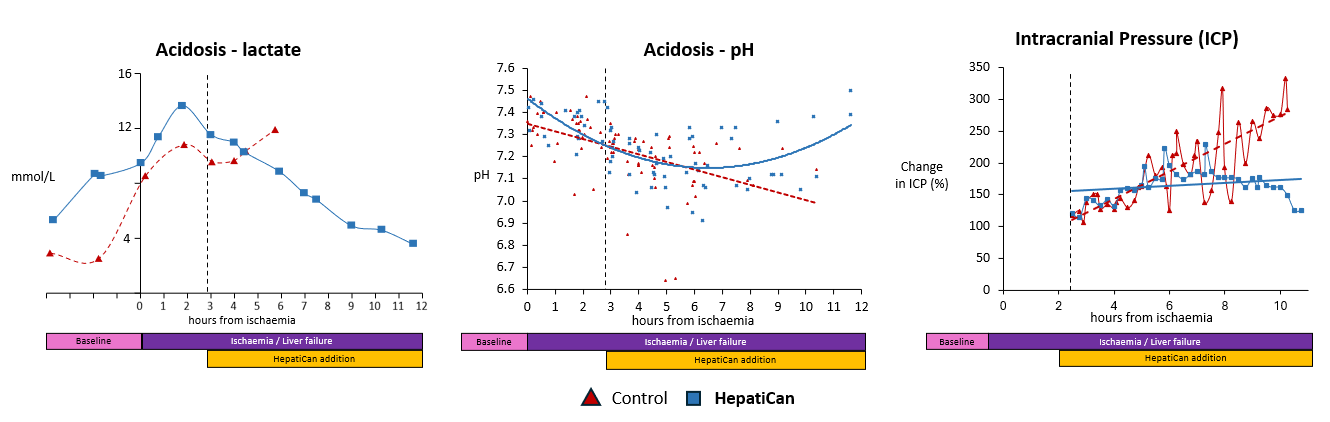
Proof of Concept

Technology
HepatiCan is used in ITU setting where it is connected to the patient via an apheresis machine.
Patient ‘s plasma circulates through the canister containing healthy human liver cells providing a substitute liver for up to 5 days. Liver cells are able to replicate within 48-60 hours. The failed liver, given time by HepatiCan, and a nucleus of healthy cells, is then able to recover and resume normal functionality.
HepatiCan technology is protected by recent patents.
Market
Europe
Independent Health Economic assessment estimates QALY value for curative treatment of ALF patients to be 21.54 QALY’s. UK NICE reimbursable cost per QALY is approx. £30,000.
On this estimate European HepatiCan ALF mkt alone would be worth in the region of £2.7 billion.
Other indications plus Rest of World would give a mkt in excess of £20 billion.

Timeline
Competition
Only current commercial competition is High Volume Plasmapheresis (HVP).
Work is ongoing to use genetically modified porcine livers but this is still in early stages. Similar attempts with kidneys, which should be less complex, has not yet yielded a commercial product.

Team
Prof Clare Selden
Founder, CSO
Clare is a UCL Professor of Experimental Hepatology, based at the UCL’s Royal Free Hospital campus, and is Founder of Hepatican Ltd. Her research focuses on translational medicine, particularly harvesting liver cells for therapeutic gain in liver disease. Her major innovations include developing 3D organoid cultures, developing the ATMP described in this application, from scratch, cryopreserving ATMP biomass, and designing a medical device to deliver cell therapy, and testing it both in vitro and in in vivo in preclinical trials. Clare has many international collaborations. She has received over £6million research funding.
Mike Kappler
COO, SME Director
With over 30 years of experience in sales, marketing, and general management, Mike now focuses on helping companies tackle interesting and rewarding challenges. His role as Director and COO in Hepatican Ltd is helping our new SME establish itself as a key Biotech company. Most recently specializing in organ transplant solutions and devices, Mike brings relevant contacts and market knowledge to help Hepatican Ltd to revolutionize the treatment severe liver failure, he provides UK and international expertise in marketing and general management.
Steven Powell
CEO
Steven is an experienced CEO in Biotech and Medtech, specializing in company creation, growth, and investment, with equity raised across EU, USA, and China. He Led 3 IPOs, multiple M&A transactions, and served on international boards. Extensive experience in drug discovery, development, and commercialization, including biologics, vaccines, and small molecules. He has managed R&D and clinical development strategies in cancer, CNS, infectious diseases, and more. His achievements include profitable turnarounds for three companies, founding nine companies, and securing significant non-dilutive and equity financing, alongside four successful exits via sales.
Steve Gore
CFO, SME Director
Steve specialises in CFO and Non-Exec Director roles for IT, tech, and service businesses. With over 20 years of experience across UK and international markets, He began his career as a Chartered Accountant with Deloitte, later leading a successful turnaround for Europe’s largest yacht broker. He then shifted to the tech sector, serving as UK FD for a listed European Group before founding and growing a company. He now supports startups and high-growth SMEs, guiding investment rounds, sales, and MBOs to help them achieve their goals.
News